When The Average Kinetic Energy Of A Gaseous System
When the average kinetic energy of a gaseous system. When the average kinetic energy of the gas molecules goes up the temperature goes up. What is the Average Kinetic Energy of a Gas Molecule. Decreases and the molecular mass increases.
Kinetic Molecular Theory can be used to explain both Charles and Boyles Laws. Compare the average kinetic energy of the water molecules in the bottle at 7 am. In that case it is going to turn into its gaseous state.
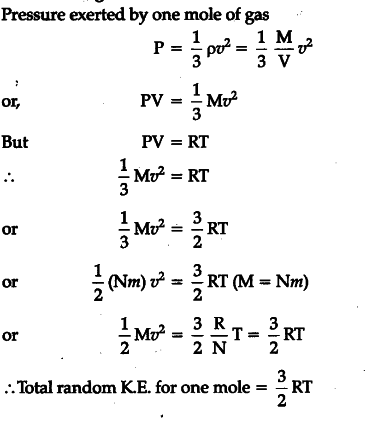
Pressure is caused by the change in momentum associated with collisions of gas. The formula for the kinetic energy of a gas defines the average kinetic energy per molecule. K average kinetic energy per molecule of gas J.
Decreases and the molecular mass remains the same. 14Which term is defined as a measure of the average kinetic energy of the particles in a sample of matter. 15Solid A at 80ºC is immersed in liquid B at 60ºC.
Is higher than at 7 am. The average kinetic energy K is equal to one half of the mass m of each gas molecule times the RMS speed v rms squared. State the direction of heat transfer between the surroundings and the water in the bottle from.
A hot object has greater average kinetic energy but may not have greater total kinetic energy. Each molecule has this average kinetic energy. K 1 2 m v 2.
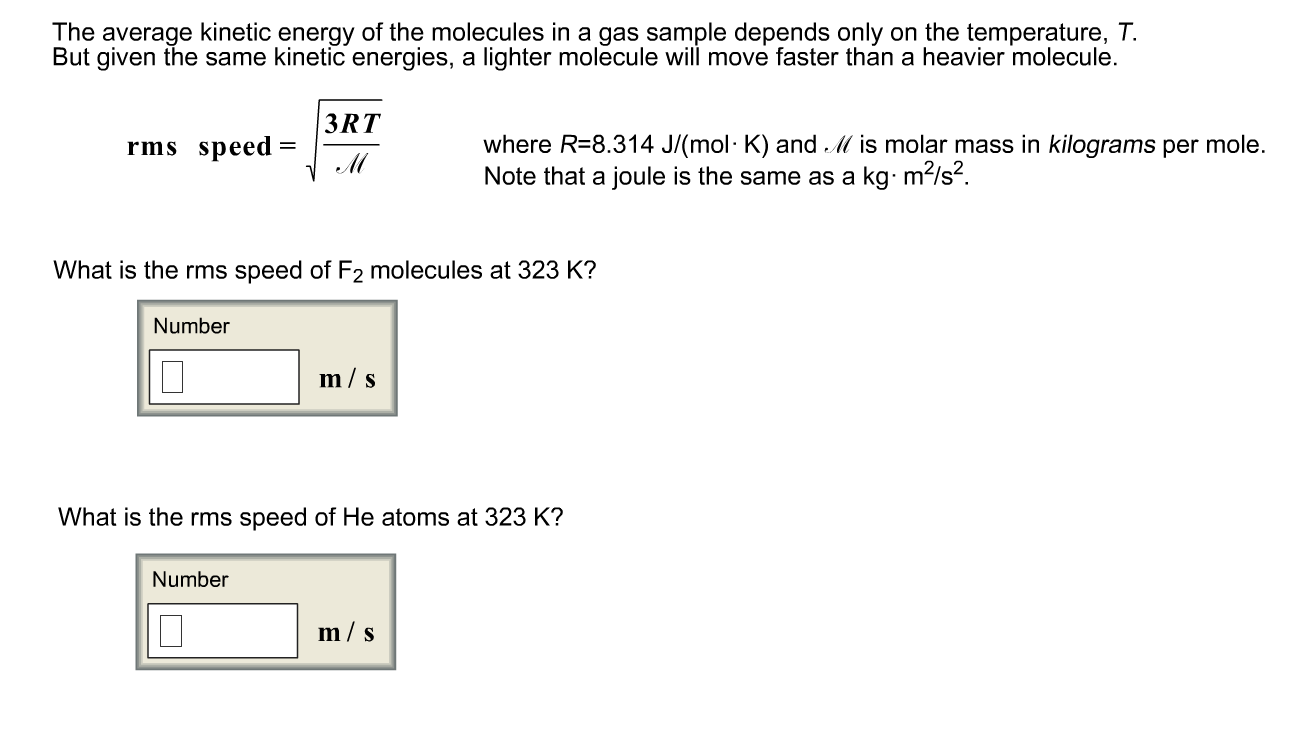
This chemistry video tutorial explains how to calculate the average kinetic energy of a gas and the root mean square velocity as well. We then extend this definition to any system of particles by adding up the kinetic energies of all the constituent particles.
V ω A 2 x 2 K 1 2 m ω 2 A 2 x 2 for x 0 K 1 2 m ω 2 A 2 K max.
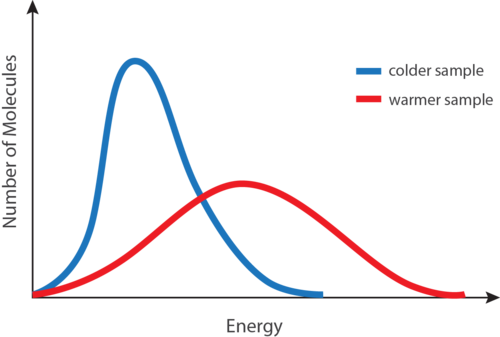
The equipartition theorem is quite general. So kinetic energy is conserved in collisions between molecules. Here is abotehr video about the overview of the gases. The relationship is based on the postulate that all gases at the same temperature have the same average kinetic energy recall that a result of the Kinetic Theory of Gases is that the temperature in degrees Kelvin is directly proportional to the average kinetic energy of the molecules. The equipartition theorem is quite general. K 1 4 m ω 2 A 2 1 c o s 2 ω t Kinetic energy varies periodically with double the frequency of SHM. What is heat in terms of kinetic energy. K 1 2 m v 2. The kinetic energy of a particle is one-half the product of the particles mass m and the square of its speed v.
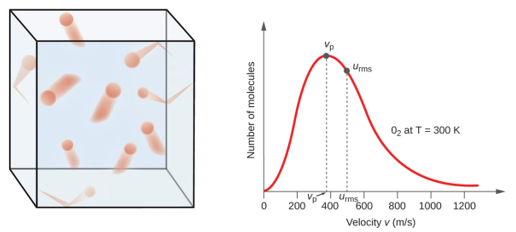
It contains plenty of. 22 2 u2 u u u12 n n The mean square speed is the direct measure of the average kinetic energy of gas molecules. The relationship is based on the postulate that all gases at the same temperature have the same average kinetic energy recall that a result of the Kinetic Theory of Gases is that the temperature in degrees Kelvin is directly proportional to the average kinetic energy of the molecules. That is very important to understand. In the liquid the motion giving rise to kinetic energy is restricted to a narrower range about the potential energy minimum than it is in the gas phase. K 1 2 m v 2. 15Solid A at 80ºC is immersed in liquid B at 60ºC.



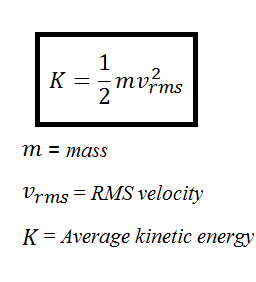






























Posting Komentar untuk "When The Average Kinetic Energy Of A Gaseous System"